 For more information, log on to-
For more information, log on to-
http://shomusbiology.weebly.com/
Download the study materials here-
http://shomusbiology.weebly.com/bio-m...
Enzyme catalysis is the catalysis of chemical reactions by specialized proteins known as enzymes. Catalysis of biochemical reactions in the cell is vital due to the very low reaction rates of the uncatalysed reactions.[citation needed]
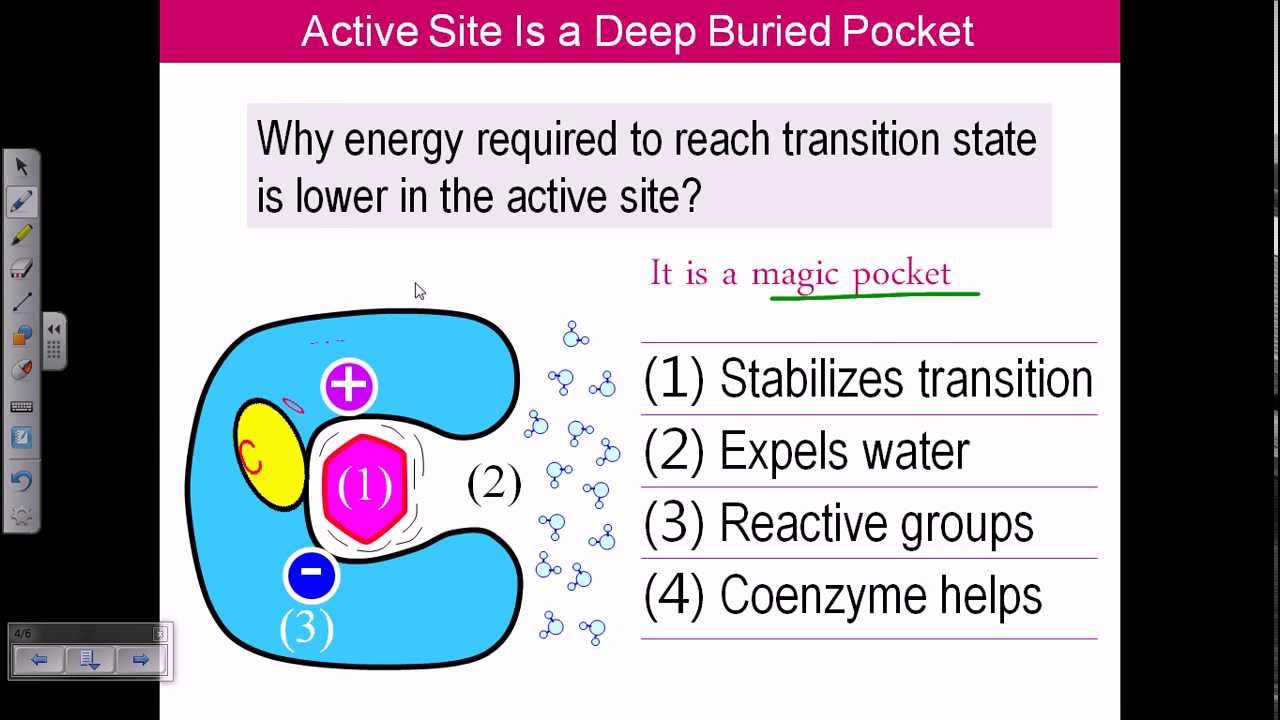
The mechanism of enzyme catalysis is similar in principle to other types of chemical catalysis. By providing an alternative reaction route the enzyme reduces the energy required to reach the highest energy transition state of the reaction. The reduction of activation energy (Ea) increases the number of reactant molecules with enough energy to reach the activation energy and form the product.
The favored model for the enzyme-substrate interaction is the induced fit model.[1] This model proposes that the initial interaction between enzyme and substrate is relatively weak, but that these weak interactions rapidly induce conformational changes in the enzyme that strengthen binding.
The advantages of the induced fit mechanism arise due to the stabilizing effect of strong enzyme binding. There are two different mechanisms of substrate binding: uniform binding, which has strong substrate binding, and differential binding, which has strong transition state binding. The stabilizing effect of uniform binding increases both substrate and transition state binding affinity, while differential binding increases only transition state binding affinity. Both are used by enzymes and have been evolutionarily chosen to minimize the Ea of the reaction. Enzymes which are saturated, that is, have a high affinity substrate binding, require differential binding to reduce the Ea, whereas small substrate unbound enzymes may use either differential or uniform binding.
These effects have led to most proteins using the differential binding mechanism to reduce the Ea, so most proteins have high affinity of the enzyme to the transition state. Differential binding is carried out by the induced fit mechanism - the substrate first binds weakly, then the enzyme changes conformation increasing the affinity to the transition state and stabilizing it, so reducing the activation energy to reach it.
It is important to clarify, however, that the induced fit concept cannot be used to rationalize catalysis. That is, the chemical catalysis is defined as the reduction of Ea‡ (when the system is already in the ES‡) relative to Ea‡ in the uncatalyzed reaction in water (without the enzyme). The induced fit only suggests that the barrier is lower in the closed form of the enzyme but does not tell us what the reason for the barrier reduction is.
Induced fit may be beneficial to the fidelity of molecular recognition in the presence of competition and noise via the conformational proofreading Source of the article published in description is Wikipedia. I am sharing their material. Copyright by original content developers of Wikipedia.
Link- http://en.wikipedia.org/wiki/Main_Page PPT source- National Taiwan University, Jaung Web. Copywright by original content developer.
Link- http://juang.bst.ntu.edu.tw/BCbasics/...
Enzyme catalysis lipids polymer | |
| 90 Likes | 90 Dislikes |
| 25,299 views views | 750K followers |
| Education | Upload TimePublished on 22 Aug 2013 |
Không có nhận xét nào:
Đăng nhận xét